Which of the Following Molecules Has the Highest Boiling Point
Cl straight chain molecule with the highest number of carbons has the highest boiling point. QUESTION 10 Which of the following molecules has the HIGHEST boiling point.
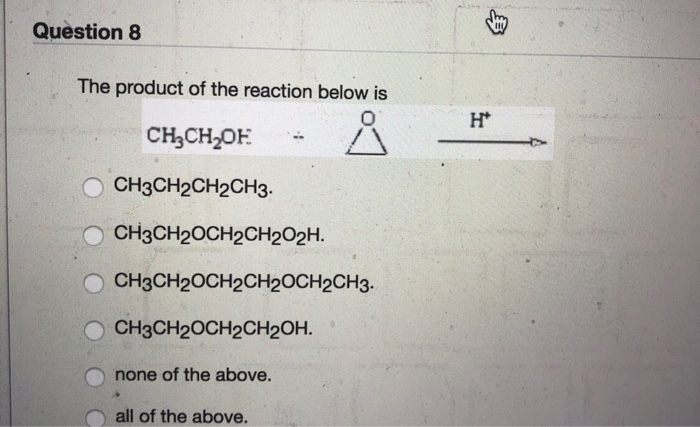
Solved Question 5 Which Of The Following Molecules Would Chegg Com
Which of the following has the highest boiling point.
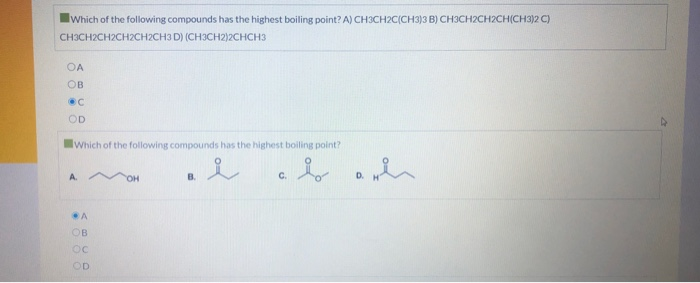
. So answer option A is correct. The more sphere like the molecule the lower its surface area will be and the fewer intermolecular Van der Waals interactions will operate. Which of the following compounds has the highest boiling point.
So it is easy to identify that NaCl sodium chloride has highest boiling point amongst the given choices. On the basis of bonding the compound which has highest boiling point is sodium chloride. It will have the next highest boiling point.
As it is spherical and there is less intermolecular contact. The hydrogen bonding in CH3CH2OH and CH3CH2CH2OH give these molecules a higher boiling point. Glucose usually melts at 146 C.
CH₃OCH₃ Dimethyl ether has a boiling point of -23 C C₂H₅OH Ethanol has a boiling point of 7837 C CH₃CH₂CH₃ Propane has a boiling point of -42 C CH₃CHO Acetaldehyde has a boiling point of 202 C C₂H₅OH Ethanol has the highest boiling point. The hydrogen bond is stronger intermolecular forces. 1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8.
Sodium chloride has boiling point 1413 C. HCl will have a. Propan-1-ol has highest boiling point because propan-1-ol contain H-bonding in their structure.
Ethane is lightest and has lowest boiling point. Another isomer of ceC10H22 246-trimethylheptane has boiling point of pu1448 mathrmoC which is also lower than that of n-nonane. Dimethyl ether CH3OCH3 is a polar molecule.
Of the two CH3CH2CH2OH has more electrons and thus stronger London forces so it is the higher-boiling compound. Branching decreases the boiling point. Water boils at 100C.
Straight chain alkyl halides have greater boiling point than their isomers. The greater the charge on the ions the stronger the forces holding them together. All of these examples means in order to predict boiling point simply by molecular weights we may need additional information such as molecules structural features etc.
Alcohol are found to have boiling point approximately as 7837 C. Rank the following compounds in order of increasing. CH4 is non-polar hence contain only weak van der waals forces hence it will have a low boiling point.
A CH3CH2CH3 B CH3-C-H C CH3-O-CH3 D CH3CH2OH 4 Which of these compounds is a properly named 2 alcohol. So C H 3 C H 2 C H 2 C H 2 Cl has highest boiling point. Chemistry questions and answers.
Which of the following molecules has the highest boiling point AnswerHF will have the highest boiling pointExplanationCH4 is non-polar hence contain only weak van der waals forces hence it will have a low boiling point. To predict boiling point we should have idea about van der waals forces which depend on surface area of the given mole View the full answer Transcribed image text. Not at all Slightly Kinda Very much.
It has the highest boiling point that is 7778 C173 F Solve any question of Haloalkanes and Haloarenes with-. Boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure surrounding the liquid. Which of the following nonpolar molecules has the highest boiling point.
As branching increases boiling point decreases. 1- butanol butanal pentanec. Boiling points increase as the number of carbons is increased.
Which of the following compounds are listed in order of increasing boiling pointa. Methanol has strong hydrogen bonds. Therefore the element with the greatest total number of electrons will have the highest boiling point iodine and the element with the smallest total number of electrons will have the lowest boiling point hydrogen.
D CH3C-H 3 Which of the following molecules has the highest boiling point. It has the highest boiling points Next comes methanol CH4O or CH3OH. Urea is an organic compound widely used as a fertilizer.
The boiling points of the given compounds are as follows 1 2-Pentanone 101 C 2 1-Pentanol 138 C 3 Painting 361 C 4 Painting 103 C. Substance A is nonpolar substance B is polar and substance C undergoes hydrogen bonding. CaCO3 is an ionic compound.
2pentanone pentanol pentane and pentanal THIS IS THE BEST ANSWER 1-Pentanol has the highest boiling point. Cl is butyl chloride. HCl will have a lower boiling point than HF since F is more electronegative than Cl and possess a greater degree of hydrogen bonding.
Option D Sodium Chloride. Rank the following compounds in order of increasing boiling points and explain your choice of order. Among the C7 isomers neopentane would have the next lowest bp.
P is much less electronegative than both Cl and F. Of the diatomic elements H2 N2 O2 F2 Cl2 Br2 I2 all have dispersion forces. Advertisement Survey Did this page answer your question.
Alcohol are found to have boiling point approximately as 7837 C. The C-O bond dipoles reinforce each other so the molecule has a dipole moment. AnswerHF will have the highest boiling point.
Which ionic compound has the highest melting point. Correct option is A Those with strong intermolecular forces will have high melting and boiling points. CH3CH2CH3 44 amu 01 dipole moment CH3OCH3 46 amu 13 dipole moment CH3Cl 50 amu 19 dipole moment CH3CHO 44 amu 27 dipole moment CH3CN 41 amu 39 dipole moment Click again to see term 124 Previous Next Flip Space Created by jenna_lunt9 Terms in this set 24 CH3CN.
It is water white liquid with a sharp odor. Three molecular compounds A B and C have nearly identical molar masses. Asked Jun 26 2017 in Chemistry by NVdes.
The boiling point of butane is close to 0 degrees Celsius whereas the higher boiling point of butanone 796 degrees Celsius can be explained by the shape of the molecule which creates an attractive force between the oxygen on one molecule and the hydrogen on a neighboring molecule. Pentane 1- butanol 2 butanoneb.
Which One Of These Structure Has The Highest Boiling Point Nonane Or 2 2 Dimethylheptane Quora

Chemistry Notes Spontaneity Entropy And Gibbs Free Energy In 2020 Chemistry Notes Chemistry Engineering Notes

Ap Bio Notes Hydrogen Bond Study Notes Notes
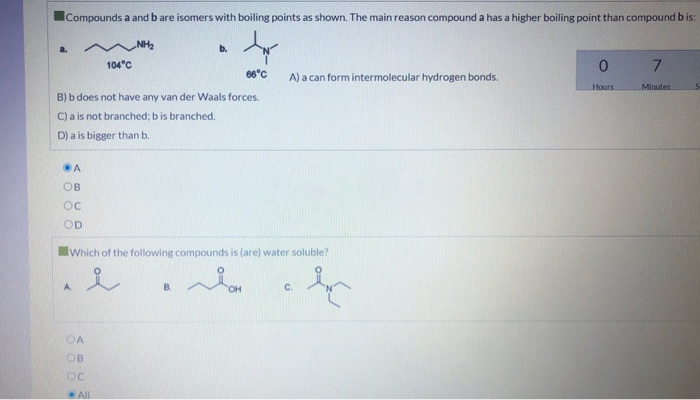
Solved Which Of The Following Compounds Has The Highest Chegg Com

Answered 16 Which Of The Following Molecules Bartleby
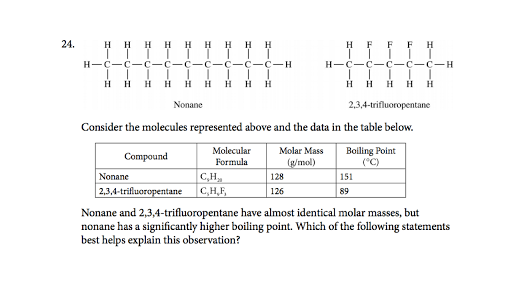
Boiling Point Comparison Ap Chemistry Multiple Choice Video Khan Academy

Boiling Melting Points And Intermolecular Forces Youtube

Solved Which Of The Following Compounds Has The Highest Chegg Com

What Temperature Does Water Boil At Boiling Point Elevation Compound Interest Physical Chemistry Chemistry High School Chemistry
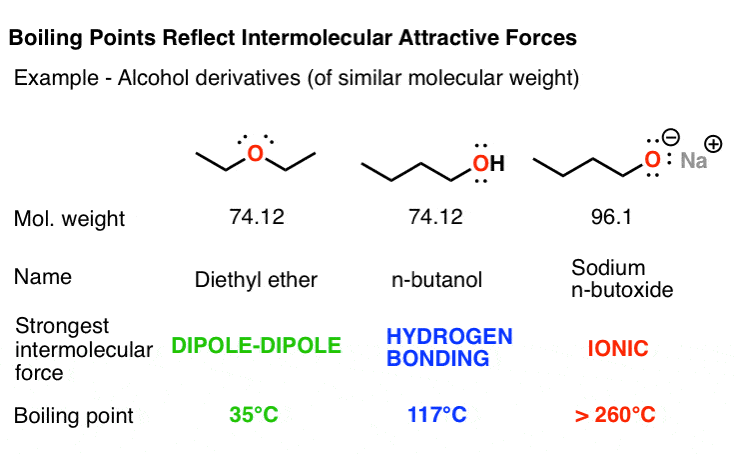
3 Trends That Affect Boiling Points Master Organic Chemistry

Solved Question 5 Which Of The Following Molecules Would Chegg Com

Pin On Structure And Bonding In Organic Chemistry
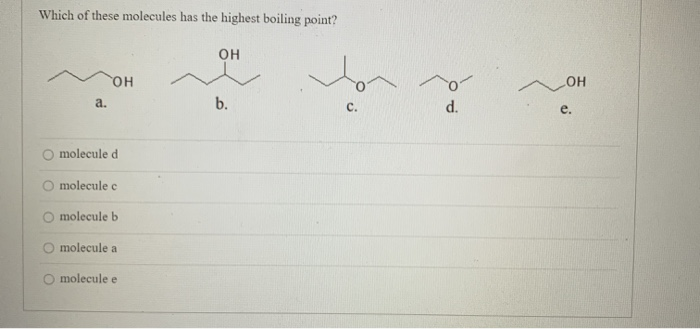
Solved Which Of These Molecules Has The Highest Boiling Chegg Com

Which Of The Following Has The Highest Boiling Point I 2 Methyl Pentane Ii 2 3 Dimethyl Youtube

Chemistry Notes Spontaneity Entropy And Gibbs Free Energy Chemistry Notes Chemistry Chemistry Basics
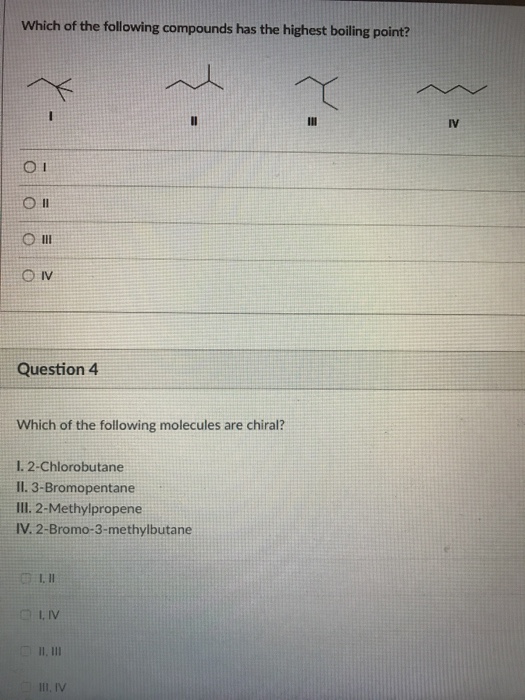
Solved Which Of The Following Compounds Has The Highest Chegg Com
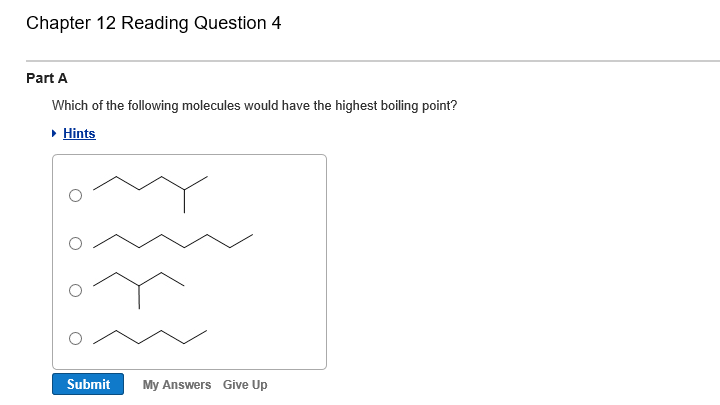
Solved Which Of The Following Molecules Would Have The Chegg Com
Solved Please Help With Both Questions 3 Rank The Following Chegg Com

Intermolecular Forces How Can I Determine The Highest Boiling Point Given A List Of Molecules Chemistry Stack Exchange
Comments
Post a Comment